Sample Requirements and Instructions
1. SAMPLE REQUIREMENTS
Sample quality directly impacts test quality and subsequent analysis.
Therefore, Dante Labs has extensive sample quality control procedures to ensure submitted samples conform to requirements for downstream processing.
To guarantee the normal processing of your project, samples should meet the standards given below. If your samples do not meet these standards, and you are unable to produce higher-quality samples, please contact us at customer.support@dantelabs.com before shipping your samples.
Notes:
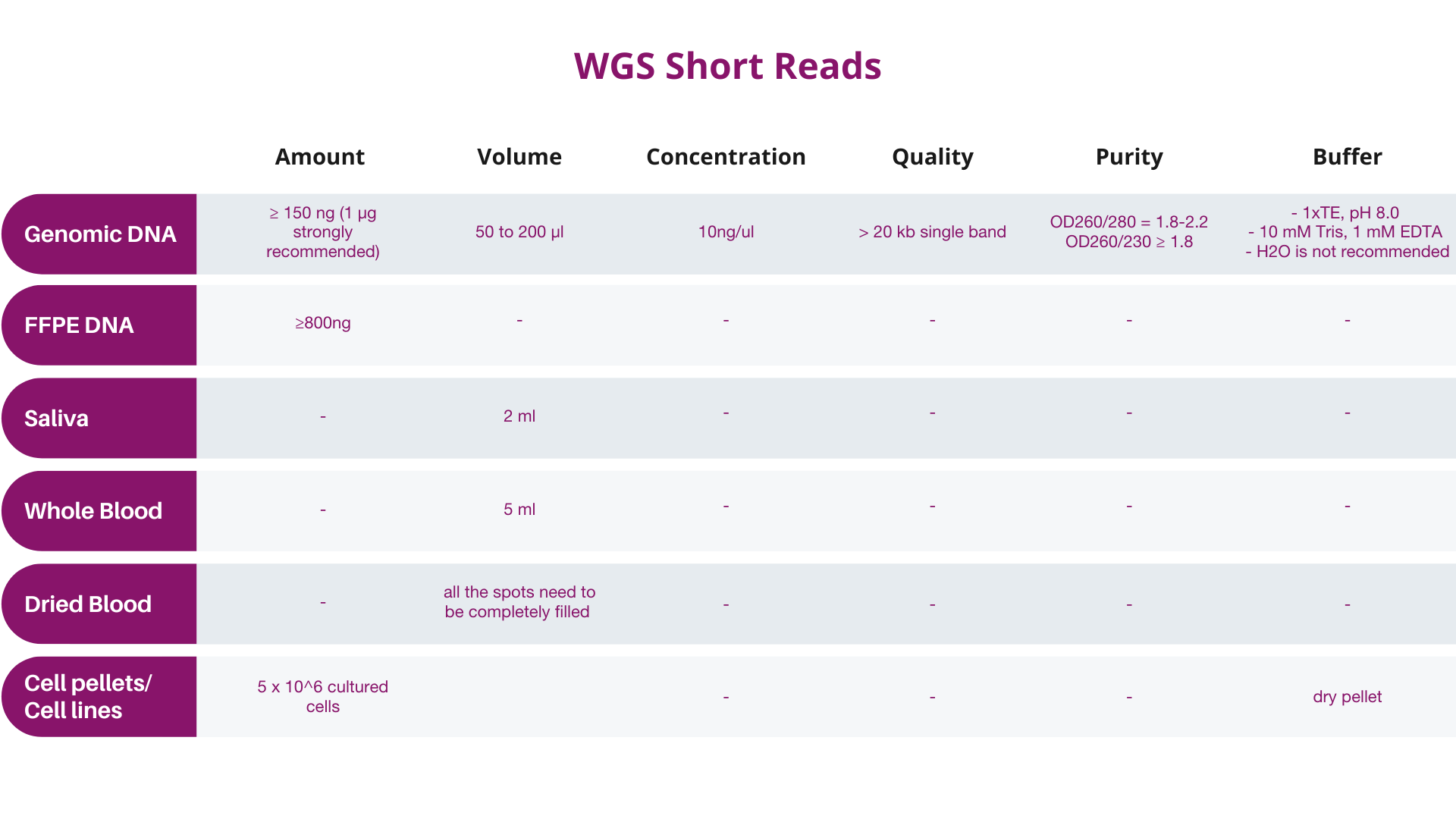
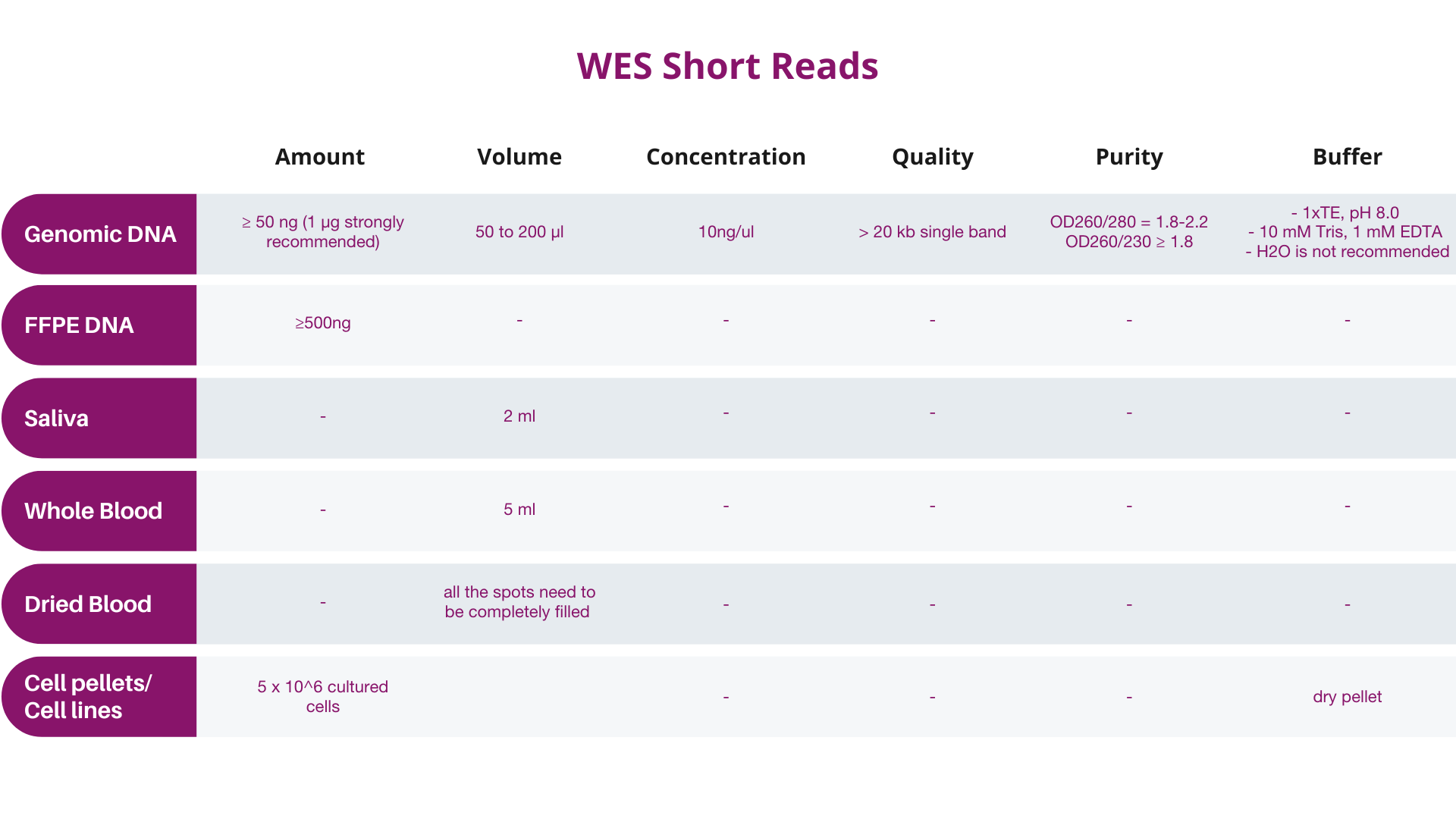
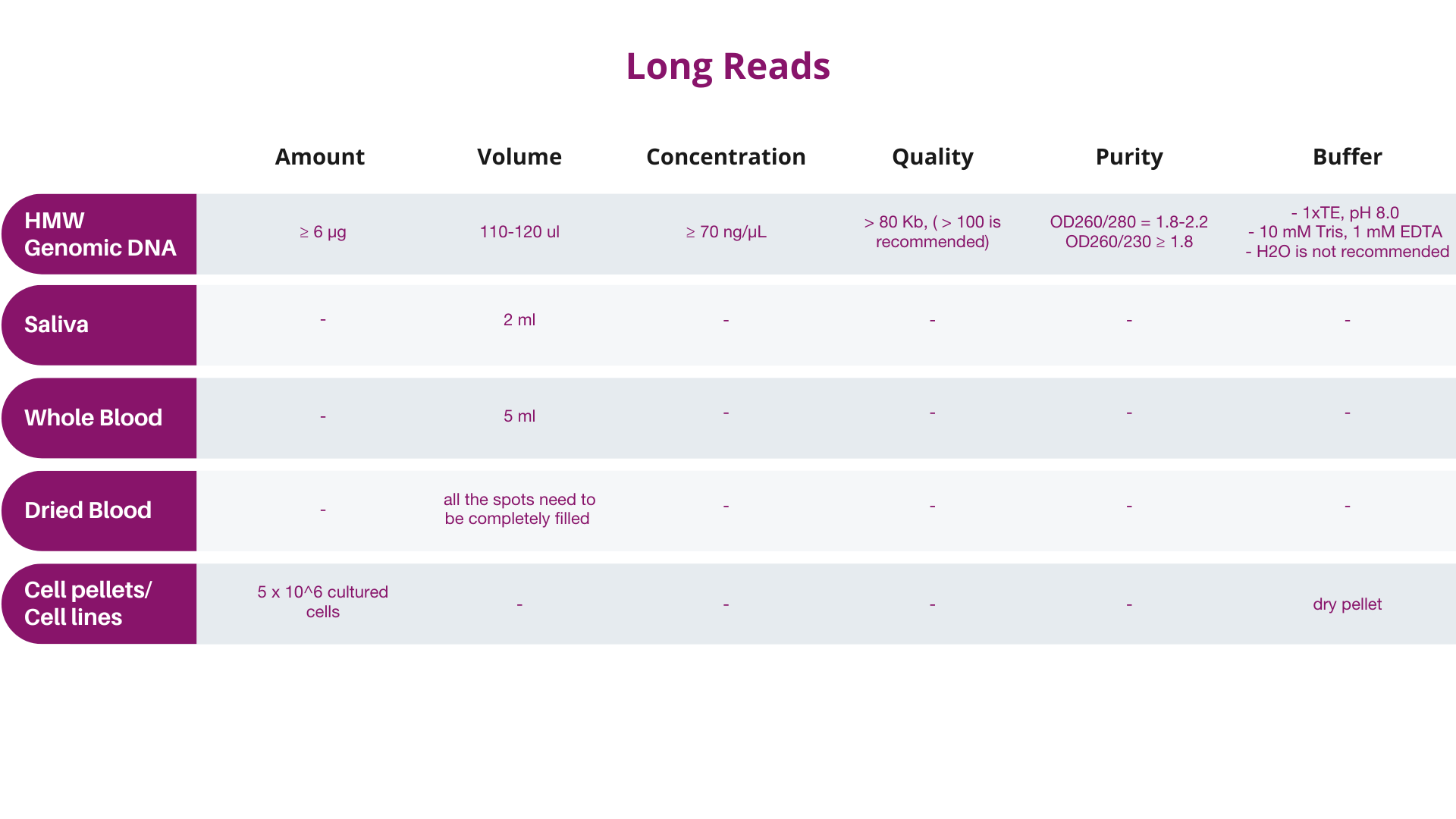
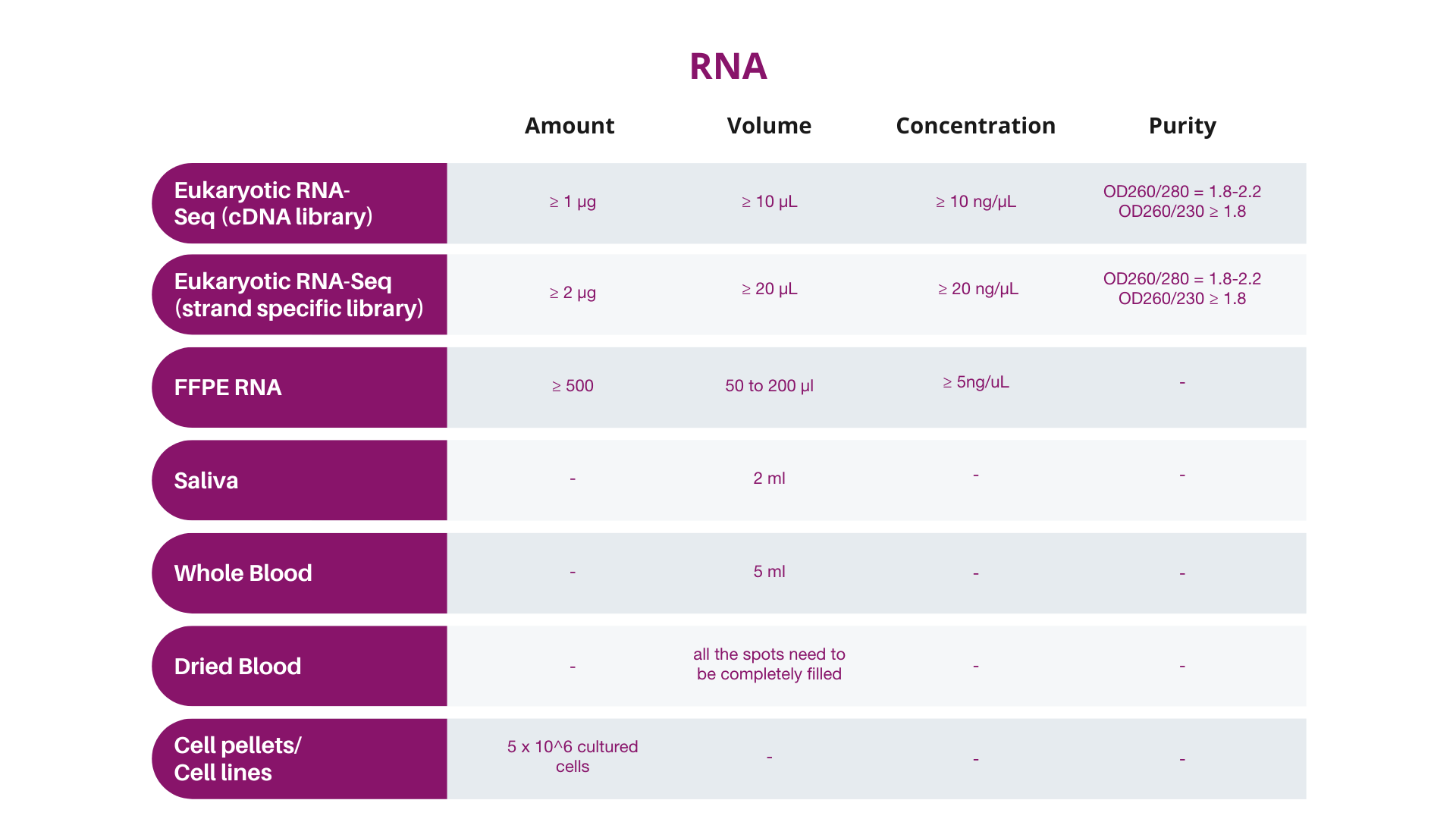
A. Input quantity should be determined preferably by Qubit® instead of by NanoDropTM, and the final quantity and concentration should conform to Dante Omics' specifications.
B. Samples not meeting these specifications should be designated as “at risk” by the customer, and will be subject to billing regardless of data quality.
Please contact us at customer.support@dantelabs.com for further details.
2. PRE-QUALITY CONTROL (QC) INSTRUCTIONS
Quality of extracted DNA or RNA might affect the results of the analysis, therefore it is required to the customers to provide the results of sample quality obtained using one of the following methods:
- Qubit
- NanoDrop
- Agarose gel electrophoresis
- Agilent 2100
It is recommended samples be analyzed by Qubit (with quantity indicator), so that the results will correspond more closely to Dante Omics QC results. NanoDrop quantification is NOT recommended. If NanoDropTM is utilized for pre-QC quantification, Dante Omics strongly recommends that you send more DNA/RNA for processing than the amounts given above (10μL more of the recommended amount). Dante Omics will perform an extra QC before the analysis.
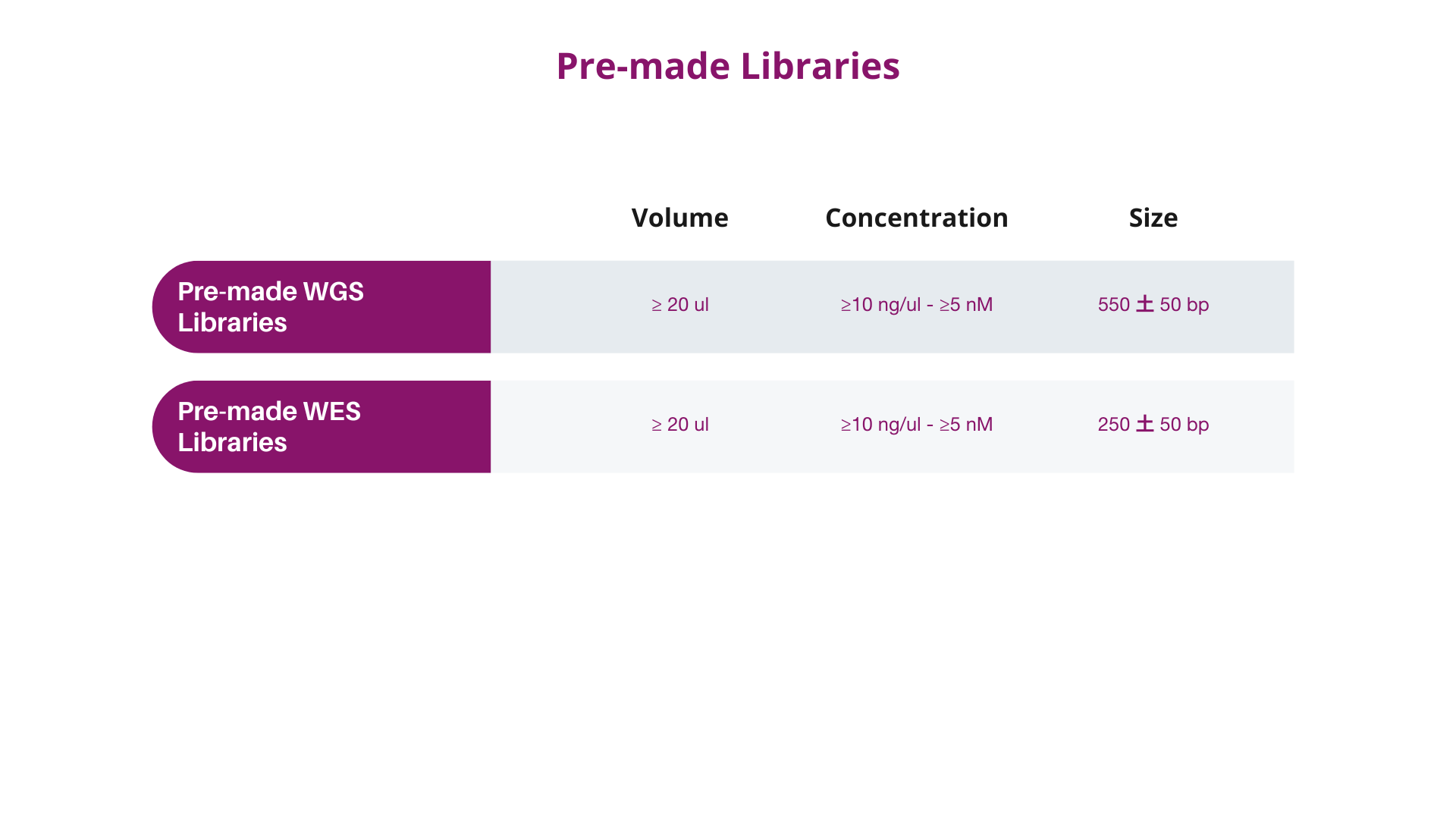
3. LIBRARY REQUIREMENTS (IF NEEDED)
In case you intend to ship ready-made libraries to Dante Omics, please take into consideration the following parameters to be met, depending on the type of libraries.
4. SAMPLE LABELING RECOMMENDATIONS
It is important to prevent inappropriate naming of the samples. Dante Omics do not require any personal information to be placed on the samples. The best option for naming the samples for us would be if the customer assign to each specific sample a 10 characters barcode where:
A. The first three characters are capital letters
B. The following seven digits are numbers (please do not use any 0)
In the case the customer doesn’t provide that format of labeling, we will assign internal barcodes to the samples as the samples are accepted by the laboratory as part of our internal processes.
5. SAMPLE PACKING RECOMMENDATIONS
- For DNA and RNA samples, Dante Omics accepts 1.5 ml or 2 ml flip-top DNase- and RNase-free microcentrifuge tubes. Please use Parafilm to seal each tube before packaging. Dante Omics does not recommend shipping samples dissolved in organic solvents (such as absolute ethanol or isopropanol) because the solvents may cause leakage of the samples, which can result in cross-contamination between samples. If it is unavoidable to ship samples in organic solvents, please use flip-top tubes and seal the opening of the tube with at least 10 layers of Parafilm.
- In order to avoid crushing during shipping, Dante Omics highly recommends placing the sample tubes in a container such as a 50-ml tube or a box with interior racks/holders. Cotton and
absorbent papers can be used to prevent tubes from moving around inside the container.
- RNA, Libraries or pools of Libraries samples should be kept in dry ice during shipment. DNA samples can be shipped at 2-8 °C.
Saliva samples should be shipped at room temperature.
- In order to stick with our high quality control standards, 96-well plates and PCR stripe tubes are NOT acceptable containers for your sample shipping. The only container we allow for sample shipping is a 1.5 ml or 2 ml tube with flip-top. (See picture below).
6. SHIPPING SAMPLES TO DANTE LABS
Disclaimer: The information below only constitutes a recommendation for shipping samples classified as “non-regulated materials” to our facility. At the time this document was prepared, gDNA/total RNA was not defined as a diagnostic specimen in the International Air Transport Association (IATA) packing instructions, and therefore no special packaging requirements are listed. Due to continuing changes in regulations, customers should always check with their safety office and/or shipping department to ensure regulatory compliance.
1. Ensure that all samples conform to our quality standards and that they are prepared and packaged according to the guidelines given above
2. Select a reliable courier and choose the priority option for shipment. Dante Omics recommends FedEx (fedex.com), UPS (ups.com), or DHL (dhl.com). For domestic shipping to our Italian lab, we recommend using Priority Overnight (arriving next day by 10:30 am) or Standard Overnight (arriving next day by 3:00 pm) from FedEx, OR Next Day Air Saver (arriving next day by 3 pm) from UPS.
7. SAMPLE TRANSPORTATION OPTIONS
DNA
- Lyophilize the DNA for shipping at ambient temperature
- Pack with ice packs/blue ice (2-8 °C)
- Use the cold-chain transportation system (2-8 °C) of the courier
- DNA Stable (Liquid format, Biometrica)
- Pack gel pack ice (2 °C – 8°C)
RNA
- Lyophilize the RNA for shipping at 2-8 °C or ambient temperature
- Suspend RNA in TE or water and ship on dry ice
- RNAstable (Biomatrica)
- Pack in dry ice (-60 °C – -80 °C)
Notes:
A. It is highly recommended that RNA samples be shipped in dry ice packaging. Other packaging/transportation methods may add impurities or cause slight degradation of the RNA.
B. The quantity of dry ice and ice bags needed varies with the season (i.e., room temperature), transit time, and the thickness of Styrofoam box and receptacle.
Please contact your local courier office for estimated transit time. Normally, dry ice is consumed (sublimates) at a rate of 5 kg (11 pounds) per day.
Shipping samples to EU sample collection site
Contact your local international courier and complete an INVOICE (commercial invoice, customs invoice, or pro-forma invoice) as required for customs, and include it with the shipment.
Please complete the INVOICE as below:
1. RNA or DNA Samples for Research Use Only
2. Non-Dangerous, Non-Infectious
3. No Commercial Value, Value for Customs Only
4. Declare the value of the goods for customs [i.e. $1.00 (USD) or €1.00 (EUR)]
5. Number of samples and volumes [the # of samples, and the estimated volume]
6. Type of container
Note:
DO NOT include any other information about the source or about how you packed it.
DO NOT include words such as “Human, Tissue, Cell, Blood, blue ice, dry ice, etc.” on the airway bill. Just write “RNA or DNA Samples for Research Use Only” (Please refer to the airway bill in the above picture).
DO NOT write our company name on the airway bill.
Package the samples with
1. A completed and detailed Sample Information Form
2. Include any QC data for the samples if available (Qubit/Nanodrop/agarose gel electrophoresis/Agilent 2100).
After arriving at the Dante Omics site, the Project Manager will be responsible for providing timely feedback to you on the progress of your project.
Conditions for Blood Sample Collection and Transportation
Sample quality is of paramount importance as it directly affects the quality of sequencing and subsequent bioinformatics analysis. At Dante Omics, we prioritize sample quality control to ensure that the samples submitted adhere to the necessary requirements for downstream processing.
To ensure the smooth processing of your project, it is essential that your samples meet the following standards. If your samples do not meet these standards and you are unable to obtain higher-quality samples, we kindly request you to consult with your designated Dante Omics Project Manager before proceeding with shipping your samples.
Maintaining high sample quality is crucial for obtaining accurate and reliable results, and our
team is dedicated to assisting you in achieving the best possible outcomes for your project.
SAMPLE LABELING RECOMMENDATIONS
To ensure proper sample identification, it is crucial to avoid any inappropriate naming practices. At Dante Omics, we prioritize the privacy and security of our customers' personal information, and therefore, it is not necessary to include any personal details on the sample labels.
For optimal sample naming at Dante Omics, we recommend that each specific sample is assigned a 10 character barcode by the customer.
Please adhere to the following guidelines when creating the barcode:
- The first three characters should be capital letters.
- The remaining seven characters should be numbers (excluding 0).
By following this format, you can ensure clear and standardized sample identification.
However, if you are unable to provide samples labeled in this specific format, rest assured
that Dante Omics will assign internal barcodes to the samples as part of our internal processes once they are received at the laboratory.
We understand the importance of accurate sample tracking, and our team is committed to
maintaining the integrity and traceability of your samples throughout the testing process.
Alternatively, if agreed upon, Dante Omics can provide the barcodes to be used on the sample tubes. This ensures consistent labeling and helps maintain efficient sample tracking throughout the testing process. If you prefer this option, please discuss it with your Dante Omics representative, and they will provide you with the necessary barcodes to label your sample tubes accurately. We are dedicated to accommodating your preferences and ensuring a seamless experience throughout your project.
SAMPLE PACKING RECOMMENDATIONS
- Sealing of Tubes: Before packaging, it is important to seal each tube using Parafilm. This helps to ensure proper containment and prevents any potential leakage during shipping.
- Avoidance of Organic Solvents: Dante Omics advises against shipping samples dissolved in
organic solvents, such as absolute ethanol or isopropanol. These solvents have the potential to cause sample leakage, leading to cross contamination between samples. If it is unavoidable to ship samples in organic solvents, please use flip-top tubes and securely seal the tube opening with at least 10 layers of Parafilm.
- Container Recommendation: To prevent crushing and damage during shipping, it is highly recommended to place the sample tubes in a container such as a 50-ml tube or a box equipped with interior racks or holders. Additionally, the use of cotton and absorbent papers
within the container can help stabilize the tubes and prevent movement during transit.
- Sample Type and Container: The recommended sample type is whole blood collected in an EDTA (Purple Top) vacutainer.
- Minimum Quantity: For whole blood samples, a minimum of 5mL is required.
- Storage and Transportation: Store the samples at a temperature range of 2-8 degrees Celsius (refrigerated), avoiding freezing. During transportation, it is recommended to include
cool gel packs to maintain the desired temperature range.
- Handling: Maintain aseptic conditions while storing the samples. It is essential to transport the samples to the laboratory within 24-48 hours after collection to ensure sample integrity.
- Labeling: Label the EDTA tube with the barcode provided by Dante Omics, and
register the sample into your Genome Manager account for proper sample identification and
tracking.
- Pathogen Limitations: Dante Omics cannot accept samples from patients infected with HCV,
HIV, or other Grade 3 and 4 pathogens due to safety considerations.
By following these guidelines, you can help ensure the safe and reliable transportation of
your samples to Dante Omics for accurate testing and analysis.
VENOUS BLOOD COLLECTION
Remember to follow proper infection control measures throughout the procedure and adhere to any additional guidelines provided by your healthcare institution.
1. Gather all the necessary equipment for the venipuncture procedure.
2. Ensure you are wearing a pair of clean gloves.
3. Position the tourniquet on the patient's arm approximately 3-4 inches above the
intended collection site.
4. Remember to release the tourniquet within one minute to prevent prolonged venous
congestion.
5. Instruct the patient to make a fist, facilitating vein prominence.
6. Use your index finger to palpate and trace the path of the vein you intend to
puncture.
7. Cleanse the selected site using alcohol prep pads or an appropriate antiseptic
solution.
8. Uncap the needle and inspect the syringe to ensure proper functioning.
9. Align the needle with the targeted vein and perform the venepuncture.
10. As you collect the required amount of blood, draw it into the syringe and
subsequently transfer it into an EDTA-containing tube. Ensure proper mixing by
gently rolling the tube.
11. Once blood flow is established and sufficient samples have been obtained, promptly
remove the tourniquet to restore normal circulation and minimize bleeding at the
puncture site.
12. Upon completion of the collection, place folded gauze over the needle and gently
withdraw it from the arm.
13. Apply firm pressure with the gauze over the venipuncture site until bleeding ceases.
14. Secure the collection site with a bandage.
TUMOR SAMPLE GUIDELINES
To ensure accurate molecular profiling, please follow the specimen guidelines below when submitting tumor samples.
TISSUE REQUIREMENTS:
- 1 FFPE block with the greatest tumor content
Otherwise:
- 5 FFPE unstained slide for NGS 5 μm sections (minimum)*
- Terminal 1 H&E stained slide
*Submit additional slides if tissue size is small (<30 mm2 or < 0.14 mm3), to allow a second analysis in case of unqualified sample: 6 FFPE unstained slide for NGS 5 μm sections.
Optimal tumor size is 30 mm², minimum 14 mm². Minimum volume is 0.14 mm³.
FFPE blocks or slides for tumor profiling analysis can be shipped at room temperature.
The patient material should be labeled with an identifier.
Tumor samples should be from the most recent procedure, if adequate for testing, and must be less than 6 years old.
Tumor required to be at least 25% of the sample.